Industry News
COVID-19 vaccine rollout is in full swing!
March 2021
Johnson & Johnson COVID-19 vaccine receives FDA approval
This week, the U.S. Food and Drug Administration approved Johnson & Johnson’s one-shot COVID-19 vaccine for emergency use giving the U.S. a third tool to fight the pandemic. FDA scientists confirmed the vaccine is about 66 percent effective at preventing moderate to severe COVID-19 and about 85 percent effective at preventing the most serious manifestation of the virus. This latest vaccine requires normal refrigeration storage temperatures making storing and vaccinating even more convenient. Johnson & Johnson has already shipped 4 million vaccine doses throughout the U.S. and expects to provide 20 million doses nationwide by the end of March and 100 million by summer.
Life science companies committed to diversity in COVID-19 vaccines
Ensuring racial and ethnic diversity in clinical trial development of COVID-19 vaccines is vitally important since the pandemic has taken a disproportionate toll on people of color. Diverse communities – especially Black adults – have historically lower vaccination rates and also commonly voice hesitancy about getting a COVID-19 vaccine. This is due to factors such as language barriers, distrust of medical researchers and the healthcare system, low levels of awareness and limited access to clinical trials.
Diversity within clinical trials for a COVID-19 vaccine helps ensure safety and effectiveness across populations and may increase confidence among people of color to get the vaccine. Since multicultural communities have been frequently underrepresented in past clinical trials, there has been concentrated efforts by companies like Pfizer-BioNTech, Moderna and Johnson & Johnson to increase racial diversity within their clinical trials. This helps make sure the vaccine will work on specific groups of patients and allows researchers to better understand how these groups will specifically respond to the vaccination.
Each company selected clinical trial sites in diverse communities throughout the U.S. and across the globe that were disproportionately affected by COVID-19 to ensure that individuals in communities most impacted had the opportunity to participate. Companies also partnered with community-based partners to help with diverse recruitment, transparency and addressing any vaccine hesitancy or concern. As a result, these companies had historic levels of participation in COVID-19 clinical trials from communities of color as well as some of the most diverse clinical trials in history.
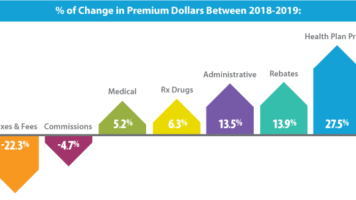
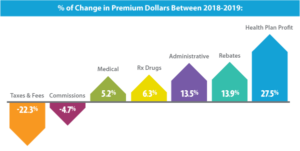
DMHC report finds health plan profits drive premium increases
The California Department of Managed Health Care (DMHC) recently released a report concluding that prescription drug expenditures are still lower than in 2017. According to the report, DMHC found that, in 2019, more of the California premium dollar went to non-medical expenses than it did prescription drugs. In fact, after accounting for manufacturer drug rebates, health plans spent almost $2.5 billion more toward non-medical expenses than for prescription drugs in 2019. In addition, while total health plan premiums increased by 5.3 percent between 2018 and 2019, the number of premium dollars that went to health plan profits increased by 27.5 percent.
For more information, contact Patty Cooper with the California Biotechnology Foundation at 916-764-2434 or [email protected].