In the News
Meet the people searching for life-saving cancer treatments
Source: Vox News
Imagine that your job is to put together an edgeless, 1,000-piece jigsaw puzzle. But you have no corner pieces to guide you, and you have no idea what the final puzzle is supposed to look like. You can only find out if you’re right by running a test on each attempt, which will tell you whether or not you’ve struck the right combination.
Every day, you rearrange the puzzle pieces in different shapes and permutations, guided primarily by what you know doesn’t work from your previous attempts. Now imagine doing that every day for five, ten, fifteen, or twenty or more years. That’s (a super simplified version of) what it’s like to be an oncology researcher at Pfizer.
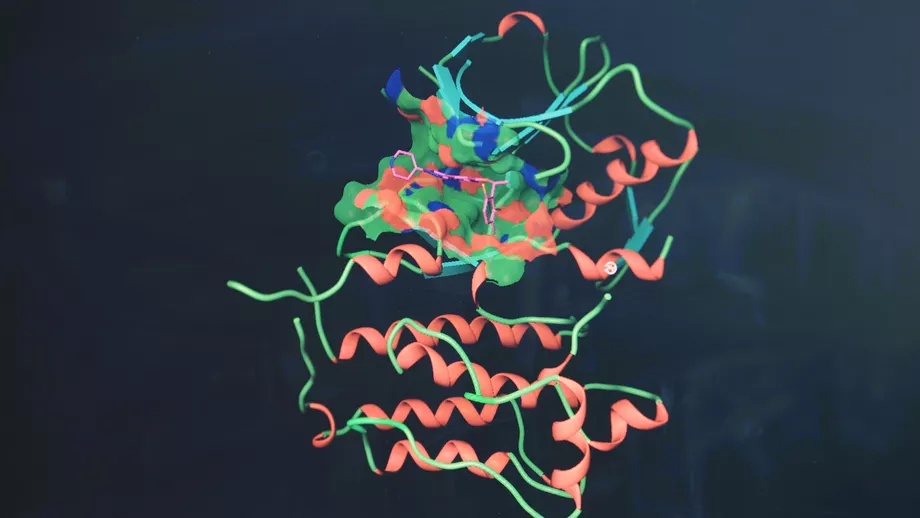
Cancer, of course, is a far more complex problem than a jigsaw puzzle. Take, for example, ALK-positive non-small cell lung cancer (NSCLC). ALK, short for “anaplastic lymphoma kinase,” is a genetic mutation that codes for an abnormal protein called tyrosine kinase. Tyrosine kinase is an enzyme that drives the growth of cancer cells. ALK-positive lung cancer, then, is a type of lung cancer that tests positive for an ALK mutation — in this case, the EML4-ALK fusion gene. About three to five percent of people with non-small cell lung cancer have this mutation.
Ted W. Johnson, Ph.D., a research fellow at Pfizer, co-designed lorlatinib, an experimental ALK/ROS1 tyrosine kinase inhibitor designed to inhibit tumor mutations that drive resistance to other ALK inhibitors and to penetrate the blood-brain barrier. Translation? It controls the “on-off switch” of ALK-positive NSCLC with very few side effects, especially compared to the debilitating side effects of traditional chemotherapy. Lorlatinib received Breakthrough Therapy status from the FDA in 2016 and is currently in phase three clinical trials.


