Industry News
Promising Alzheimer’s Disease Treatments Pose Questions about Health Care System Readiness
August 2020
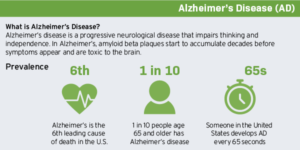
Alzheimer’s disease (AD) is a type of dementia that causes problems with memory, thinking and behavior. Symptoms usually develop slowly and get worse over time, becoming severe enough to interfere with daily tasks and eventually leading to cognitive decline and premature death. The destructive disease impacts more than 5.8 million Americans, a number that is projected to reach 15+ million by 2050 – with a cumulative cost of more than $20 trillion. Even worse, African Americans are twice as likely and Latinx people are 1.5 times as likely to develop the disease.
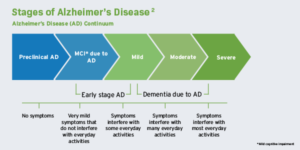
Currently there is no cure for AD. Current treatments cannot stop the disease from progressing, although they can temporarily slow the worsening of dementia symptoms and improve quality of life for those with Alzheimer’s and their caregivers. Over the past twenty years, only four new medicines were approved by the Food and Drug Administration (FDA) to treat the symptoms of AD out of 146 unsuccessful attempts.
However, there is promising news in the fight against AD, including a new blood test that may be critical to detecting AD early in patients along with significant investment in research from companies including Novartis, and Biogen. Encouraging results from recent clinical trials offer hope that one or more game-changing therapies will become available in early-2021.
Novartis is currently conducting a clinical trial program targeting cognitively healthy individuals at risk to develop symptoms of Alzheimer’s disease; and treatments like Biogen’s aducanumab may soon gain FDA approval as the first disease-modifying therapy for Alzheimer’s disease. Clinical trials evaluated patients with mild cognitive impairment associated with AD and mild AD dementia. This treatment slows cognitive and functional decline and may help preserve patients’ independence longer.
Bringing promising new treatments such as aducanumab to people living with early stage AD will require the collective effort of a wide range of stakeholders which raises many questions about whether the U.S. health care system is prepared to handle the expected millions of patients with early signs of the disease. In order to benefit from advancements in treatment, patients will need to be evaluated by specialists, undergo diagnostic testing, and be treated. A Rand Corporation Study overviews some of the concerns which include:
- Insufficient capacity of specialists and medical staff to evaluate and diagnose millions of patients for early dementia symptoms;
- Limited access and long wait times for imaging to confirm Alzheimer’s disease and to infusion centers that deliver the treatment;
- Current payment policies by insurers for novel Alzheimer’s therapies and delivery systems;
- Outdated regulatory requirements; and
- Increasing workforce capacity for the influx of candidates who qualify for new Alzheimer’s treatments.
During the 2020 Alzheimer’s Association International Conference in July, worldwide experts came together to discuss promising therapies and potential hurdles in getting millions of people diagnosed and eventually treated for Alzheimer’s disease. Two USC reports presented at the conference which assessed the hurdles to providing these treatments to people with Alzheimer’s disease in the U.S., France, Germany, Italy, Spain and the UK found identified the following obstacles:
- Funding:While all countries have or are in the process of developing national dementia strategies, none have devoted funding to implement the recommendations. Further, several AD diagnostic tests are not fully covered by health systems.
- Workforce:Primary care physicians in all countries remain reluctant to assess cognitive function because of incompatibility with their workflows, lack of training and tools, and a perceived lack of therapeutic consequences. Dementia specialists are in short supply in all countries, leading to lengthy wait times in the diagnostic process.
- Technology:Countries have limited capacity to conduct PET brain scans to diagnose Alzheimer’s disease. Not even the United States — with five PET scanners per 1 million people — would have sufficient capacity, and many patients in rural areas would face geographic obstacles.
Stay informed on the latest news and trends on the economic and health benefits of this important industry by visiting the new CABiotech.org
For more information about California’s biotechnology and life science industry, contact California Biotechnology Foundation Executive Director Patty Cooper at (916)764-2434 or [email protected].