Monthly Newsletter
Prioritizing Mental Health in an Era of Ongoing Distress
May 2022

As Mental Health Awareness Month comes to a close, now more than ever, we need to take a moment to acknowledge the sheer pain and trauma many of us are going through. Several horrific mass shootings, coupled with two-plus years of COVID-19, ongoing civil unrest and tumultuous global conflict, have left many of us feeling devastated, lost and overwhelmed.
Mental illness is a health condition just like any other health issue. More than 1 in 5 people experience a mental health condition in their lifetime. Nearly 53 million Americans have a mental illness, according to data from the National Institute of Mental Health. Here in California, almost eight million people live with a mental health condition.
Unprecedented challenges like the ones we’ve been living through have sparked continued innovation from life science companies, both large and small, working to provide treatments for conditions such as anxiety, depression, schizophrenia and post-traumatic stress disorder (PTSD). However, Mental Health Awareness Month reminds us that so much more needs to be done to support our mental health and well-being and those around us. Here are some of the ways we can help take care of ourselves and others who are hurting:
- Provide a safe place to share stressful feelings – especially for young people.
- Reach out if you believe someone you know is struggling. Listen without judgement, let them know you support them and that they’re not alone.
- Recognize the signs of people who may be struggling.
- Support policies, funding and research that prioritize mental health.
- Share mental health resources such as Mental Health America in California, NAMI California, Steinberg Institute and the National Suicide Prevention Hotline.
COVID-19 Prevention and Treatment Tips from California’s Life Science Industry
As life returns to a new normal following the COVID-19 pandemic, there are many biotech-based measures that can be taken to prevent and treat COVID-19, so it is a manageable health condition rather than a life-threatening illness. As we begin to participate in large-scale events, family gatherings and everyday activities, the following is the most up-to-date vaccine, testing and treatment options to help people stay healthy, keep others from contracting COVID-19 and stop the spread of the disease.
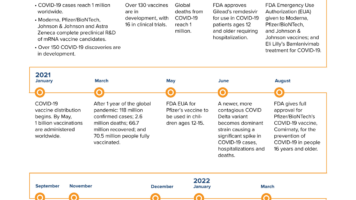
- Vaccines –Over 75 percent of Californians ages five and older are fully vaccinated. Getting vaccinated and boosted is proven to be the most effective method to prevent COVID-19, to avoid serious illness or hospitalization. The CDC recommends the following COVID-19 vaccines, including boosters:
- Testing –The availability of biotech-based PCR tests and rapid antigen tests has made it easier to control the spread of COVID-19. People can proactively take a test before entering a group setting or test and stay home when symptoms are detected. Most pharmacies carry at-home rapid antigen tests where results can be obtained in under 15 minutes. Results from PCR tests, which are 90 percent reliable, can be delivered in about 24-48 hours.
- California offers a COVID-19 testing site finder. “Test to treat” sites can be found at thislink and free at-home tests can be found here.
- Treatments – There are multiple treatment options available for people who test positive for COVID-19 and exhibit symptoms. However, treatment must be started within a few days of developing symptoms to be effective.
The CDC overviews the following COVID-19 treatments:- Paxlovid by Pfizer is an antiviral pill available for patients 12 years and older with mild to-moderate COVID-19 who are at risk for disease progression, within 5 days of symptoms onset.
- Remdesivir by Gilead is an antiviral intravenous treatment available for patients 1-month and older with mild to-moderate COVID-19 who are at risk for disease progression, within 7 days of symptoms onset.
- Bebtelovimab by Eli Lilly is a monoclonal antibody intravenous treatment available for patients 12 years and older with mild to-moderate COVID-19 who are at risk for disease progression, within 7 days of symptom onset.
- Molnupiravir by Merck is an antiviral pill available for patients 18 years or older with mild to-moderate COVID-19 who are at risk for disease progression, within 5 days of symptom onset
- Evusheld by AstraZeneca is the only pre-exposure COVID-19 treatment specifically for people who are immunocompromised. Evusheld is a long-acting monoclonal antibody intramuscular injection available for patients 12 years or older who have moderate to severe immune compromised due to a medical condition, who have not been exposed and are not experiencing symptoms
- COVID-19 treatments can be found here and here.
For more information, contact Patty Cooper with the California Biotechnology Foundation at 916-764-2434 or [email protected].