Monthly Newsletter
Life Science Industry on the Forefront of Monkeypox Testing, Vaccines and Treatments
July 2022
On July 23, the World Health Organization (WHO) declared the spread of monkeypox to be a public health emergency of international concern. The declaration will help increase awareness and unlock more financial resources and increase the global collaboration of nations under the international health regulations.
Monkeypox is related to the virus that causes smallpox. Monkeypox symptoms are similar to those associated with smallpox, but they’re not as severe; the disease is rarely fatal. Someone infected with monkeypox can experience flu-like symptoms—such as fever, muscle aches, headaches, swollen lymph nodes, and chills—and a blister-like rash, according to the U.S. Centers for Disease Control and Prevention (CDC). Some people get a rash across large areas of their body, while others get only a few lesions or blemishes in non-obvious places.
Since early May, more than 19,000 cases of monkeypox have been identified in 76 countries, with at least 434 confirmed cases here in California as of July 21. Most cases in the current outbreak have resolved without hospitalization or the need for medication. As of July 20, there have been five deaths, all of them in Africa.
The good news is that there are effective tests, vaccines and treatments which are already being distributed around the world—thanks in large part to life science company discoveries in coordination with public health efforts and previous investment in smallpox research and medical preparedness.
At least five commercial laboratories are now authorized in the U.S. to perform monkeypox tests, which will greatly increase the capacity to detect the monkeypox (orthopoxvirus) virus. Aegis Science, Labcorp, Mayo Clinic Laboratories, Quest Diagnostics, and Sonic Healthcare will now conduct the tests with many more biopharma testing options on the horizon. If an individual suspects monkeypox, a primary care physician, urgent care center or health clinic can swab a lesion and order a monkeypox test.
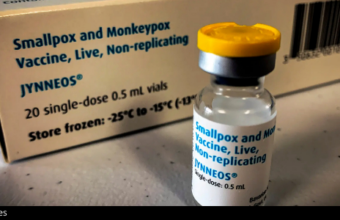
There are two vaccines that can prevent monkeypox infection: the ACAM2000 smallpox vaccine and the Jynneos smallpox and monkeypox vaccine. The CDC says people with known exposure to someone who has monkeypox should get vaccinated. When given within four days of exposure, the vaccine can prevent disease entirely. It can also lessen symptoms if given within two weeks of exposure.
There is no drug specifically approved to treat monkeypox. Doctors can prescribe antivirals used to treat smallpox (the most common is called TPOXX). However, only certain high-risk monkeypox patients—including young children, pregnant people, immunocompromised individuals, and people with serious rashes—need antivirals. Most people get better on their own in a few weeks and can control symptoms using anti-itch treatments and painkillers.
The CDC recommends the following actions to prevent monkeypox transmission:
- Avoid any skin-to-skin contact with people who have a rash that looks like monkeypox.
- Do not handle or touch the bedding, towels, or clothing of a person with monkeypox.
- Wash your hands often with soap and water or use an alcohol-based hand sanitizer.
If you are diagnosed with monkeypox:
- Isolate at home. If you have an active rash or other symptoms, stay in a separate room or area away from people or pets you live with, when possible.
For more information about monkeypox prevention, symptoms, vaccines and treatments visit the CDC’s website
For more information, contact Patty Cooper with the California Biotechnology Foundation at 916-764-2434 or [email protected].