Industry News
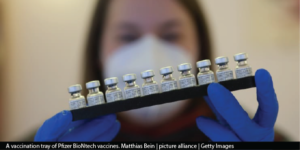
COVID-19 Vaccines Are Here!
January 2021
Since the end of last year, huge strides have been made in the search and distribution for COVID-19 vaccines. Two vaccines – one from Pfizer-BioNTech and another from Moderna – received FDA approval. Very soon after, the vaccines were distributed to all 50 states. As of February 3, 2021, nearly 55.9 million doses have been distributed and over 33.9 million have been administered to people in the U.S. In addition to the current 400 million vaccine supply, the federal government is working to buy 200 million more doses of COVID-19 vaccines, which could provide enough doses to fully inoculate nearly every American by the end of the summer.
The need for these life-saving vaccines and other virus interventions is urgent. As of February 3, 2021, the U.S. surpassed 26.2 million COVID-19 cases with more than 445,264 deaths. California has had the most COVID-19 diagnoses with 3,281,271 confirmed cases and 41,811 deaths, which have disproportionately impacted communities of color.
From pioneering innovations in testing, treatments, vaccinations and other therapies, the life science industry and public health officials are doing everything possible to curb the pandemic and get our lives back to normal. The following are a few of the thousands of innovations used to stop the spread of COVID-19:
- Pfizer plans to deliver 200 million doses of its COVID-19 vaccine to the U.S. by May. The company also said it could potentially deliver 2 billion doses globally by the end of this year.
- Novartis is joining forces with Pfizer and BioNTech to help produce COVID-19 vaccines.
- Johnson & Johnsonplans to have 100 million vaccines for Americans by the spring and would be the first vaccine given in a single shot, without requiring a booster.
- Gilead Sciences reports its antiviral remdesivir should be effective against the new, more contagious COVID-19 variants discovered in the U.K. and South Africa. Dozens of other promising treatments to combat COVID-19 or lessen the symptoms are also in the FDA pipeline.
- Eli Lilly reports its monoclonal antibody treatment cuts hospitalizations by 70% for high-risk COVID-19 patients.
Vaccinate, Vaccinate, Vaccinate.
Safe and effective vaccines are available in California. While vaccines are being delivered to our front-line workers, vulnerable populations and people 65 and older, the rest of us will soon get our turn so we all need to be prepared and proactive. Now we need to spread the word far and wide for people to sign up to get their vaccination.
Tell your constituents, family, friends, co-workers and neighbors there are multiple resources to learn more about vaccine safety and where they can sign up to receive their vaccination. They can visit Covid19.ca.gov to learn about how to continue to stay safe during the pandemic and visit myturn.ca.gov to sign up for a notification for when they are eligible for the vaccine. Or they can go to VaccinateCA to track vaccine availability in a specific area.
Despite COVID-19, FDA Approves Dozens of Treatments to Advance Patient Health
During a year of unprecedented pandemic response, the life sciences industry not only developed COVID-19 vaccines, tests and treatments, they also continued research and development of novel treatments that patients need. According to a recent report from the FDA Center for Drug Evaluation and Research, the following are some of the leading breakthroughs which were FDA approved in 2020:
- Rare Diseases:Among last year’s approvals were 31 new therapies for rare diseases. These new medicines include treatments for devastating neurodegenerative diseases such as spinal muscular atrophy and Duchenne muscular dystrophy, dangerous inflammatory conditions such as hereditary angioedema, and several rare and deadly forms of cancers.
- Infectious Diseases: In addition to last year’s approvals for treatments and vaccines for COVID-19, 2020 also saw continued progress against HIV with a first-in-class therapy for patients living with HIV who cannot manage the disease with available antiretroviral medications due to resistance, intolerance, or safety considerations. Two treatments for Ebola infection and severe Malaria, which previously lacked treatment options in the U.S., also became available to patients in 2020.
- Cancer Treatments: The FDA approved many new treatments in 2020 to address a vast array of cancers, including lung, thyroid, breast, urothelial, colorectal, prostate, bladder, leukemia and many rare forms of cancer.
- Expanded Use of Previously Approved Treatments: In 2020, the FDA approved new uses of several existing cancer medicines to treat different forms of cancer, including bladder, colorectal, kidney, prostate, lung and malignant pleural mesothelioma. In addition, a medicine originally approved to treat a form of leukemia was approved to treat relapsing forms of multiple sclerosis. A new injectable form of a multiple myeloma treatment was also approved. And three previously approved diabetes medicines were approved for patients with chronic heart failure.
For additional details, view the report or visit the FDA website.
For more information, contact Patty Cooper with the California Biotechnology Foundation at 916-764-2434 or [email protected].