Monthly Newsletter
Biotech Supports Patients During Crohn’s and Colitis Awareness Week
December 2023
On behalf of the California Biotechnology Foundation, we want to thank California policymakers, their staff, and all healthcare stakeholders who advocate each day for medical breakthroughs and for the health and lives of all Californians. Be sure to check out the U.S. Food and Drug Administration’s Novel Drug Approvals for 2023 where you will find the latest life science treatments for cancer, Alzheimer’s, heart disease mental health, COVID-19, rare diseases, and several others – many of them discovered by California-based facilities.
We truly enjoyed working with so many of you this year during our events and briefings, and we look forward to the year ahead in future collaborations with your offices. Be on the lookout for an invitation to our 2024 California Life Sciences briefing that will be held in the first part of the new year.
Wishing you a joyful and healthy holiday season and a very happy new year!
Biotech Supports Patients During Crohn’s and Colitis Awareness Week
Crohn’s and Colitis Awareness Week is observed December 1 -7 each year to shed light on the challenges and life-disrupting impacts faced by people who suffer from these illnesses.
Crohn’s disease and ulcerative colitis are painful, medically incurable, inflammatory bowel diseases (IBD) that attack the digestive system, causing symptoms including abdominal pain, persistent diarrhea, rectal bleeding, fever, and weight-loss. The condition impacts over 3 million Americans and nearly 400,000 Californians each year. About 25 percent of people with IBD will be diagnosed before their 18th birthday.
Life science innovations are offering patients hope. In fact, biologic medicines have become the standard of care for people with moderate to severe IBD and are shown to be very safe and effective. The latest biologics work by blocking chemical messages from the immune system that trigger a surge of inflammatory events in people with IBD. There are a variety of treatments available to help improve patients’ quality of life, including:
- Tumor necrosis factor-alpha blockers, which were the first class of FDA-approved biologics to treat by blocking a small protein that causes inflammation in the intestine. Examples include Remicade® (infliximab), Humira® (adalimumab), Simponi® (golimumab) and Cimzia® (certolizumab pegol).
- Integrin blockers, which prevent white blood cells that cause inflammation from entering the GI tract include Entyvio® (vedolizumab) and Tysabri® (natalizumab).
- Interleukin blockers, such as Stelara® (ustekinumab) target interleukin-12 and interleukin-23, two proteins associated with inflammation in the GI tract.
To learn more about Crohn’s and Colitis Awareness Week, check out the resources from the Color of Crohn’s & Chronic Illness (COCCI) and Connecting to Cure Crohn’s and Colitis (CtoC).
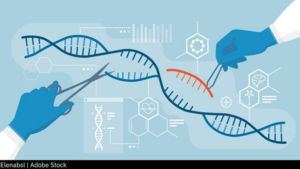
World’s First CRISPR Medicine Approved in UK for Sickle Cell, Beta Thalassemia
Great news for patients with sickle cell disease! The U.K. Medicines and Healthcare Products Regulatory Agency has conditionally approved the CRISPR gene-editing therapy, exagamglogene autotemcel (brand name Casgevy), for sickle cell disease (SCD) and beta thalassemia. This is the first time a drug built with the Nobel Prize-winning CRISPR technology has won regulatory clearance anywhere in the world.
The pioneering treatment was developed by Vertex Pharmaceuticals and CRISPR Therapeutics for people 12 years of age or older who have SCD and the recurrent pain crises or transfusion-dependent beta thalassemia severe enough to require regular blood transfusions.
SCD is an inherited disorder that affects red blood cells. People with the disease produce a faulty version of hemoglobin — the protein that carries oxygen throughout the body. These faulty cells can become misshapen and rigid, making it difficult for them to pass through small blood vessels, slowing or blocking blood flow. This in turn can compromise oxygen delivery to different tissues and organs, causing damage and inflammation. Blood vessel blockage also can result in episodes of sudden and severe pain, known as vaso-occlusive crises.
Casgevy is built from a patient’s own stem cells that are collected, edited with CRISPR in a laboratory and then reinfused. The edit allows the reinfused cells to produce a functional form of hemoglobin. Since CRISPR edits the stem cells’ DNA, the curative benefits offered by Casgevy could, in theory, last for life.
The approval is a scientific milestone for CRISPR technology which uses a viral defense mechanism borrowed from bacteria to create “genetic scissors” that can precisely alter DNA. The technology has transformed the field of genetic medicine, offering researchers and the biopharma industry a powerful tool to target the roots of inherited diseases.
Stay informed on the latest news and trends on the economic and health benefits of this industry by visiting CABiotech.org
If you have any questions about hosting informational briefings for your colleagues serving in the legislature, contact California Biotechnology Foundation Executive Director Patty Cooper at (916)764-2434 or [email protected].